A New Approach To Achieving Central Venous Access In Patients With Venous Occlusions

Information for Patients Requiring Central Venous Catheters for Hemodialysis, Nutritional Support or Cancer Therapy.
What are Central Venous Occlusions?
Upper body venous occlusions or central venous stenosis is the narrowing or complete blockage of the large veins in the chest which drain blood from the arms and head back to the heart.
WHEN DO VENOUS OCCLUSIONS OCCUR?
- Patients who have a catheter (tube) inserted into the jugular or subclavian veins can develop venous occlusions.
- It most commonly develops in dialysis patients with hemodialysis catheters.
- Venous occlusions can also occur in patients with a pacemaker in place and is worse when there is a dialysis access in the arm on the same side.
WHAT HAPPENS WHEN VENOUS OCCLUSIONS DEVELOP?
- In patients with an arteriovenous fistula or graft for hemodialysis, patients may develop swelling of the arm which can interfere with daily tasks or work and may make it difficult to use the dialysis access because of the swelling. They may also develop swelling of the face or neck.
- For patients requiring central venous catheters, having venous occlusions can make it difficult to place or replace a catheter.
- If a patient has a venous occlusion and their catheter needs to be replaced, it may require a potentially lengthy and more risky procedure (recanalization) or the placement of catheters via a vein in the leg (femoral catheter) which is associated with an increased risk of complications and patient inconvenience compared to central venous catheterization.[1]
[1] Data on file at BVT
WHAT IS THE SURFACER® SYSTEM?
 The Surfacer® System is intended to obtain central venous access to facilitate catheter insertion into the central venous system for patients with upper body venous occlusions or other conditions that preclude central venous access by conventional methods.
The Surfacer® System is intended to obtain central venous access to facilitate catheter insertion into the central venous system for patients with upper body venous occlusions or other conditions that preclude central venous access by conventional methods.
A UNIQUE APPROACH
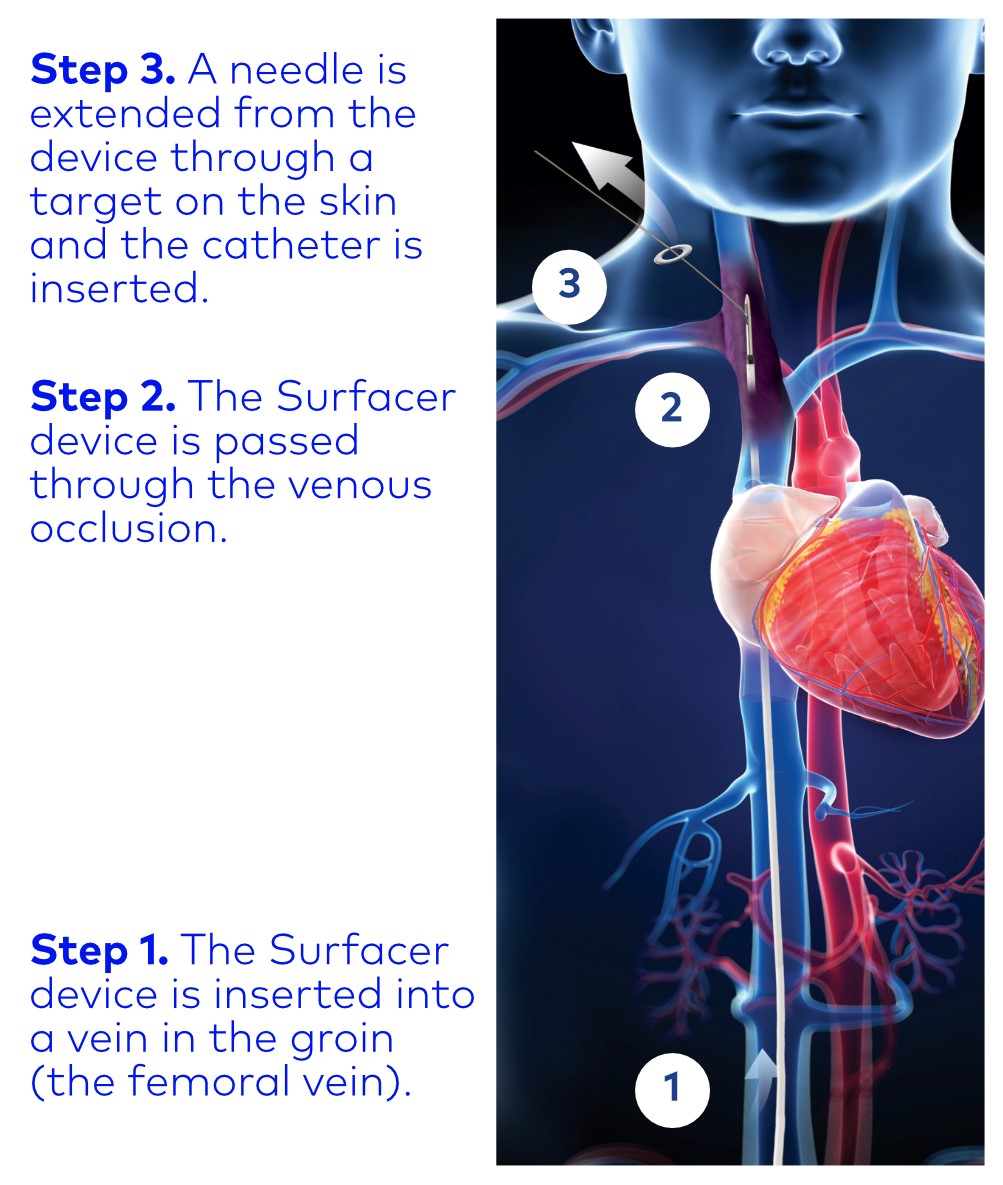 The Surfacer System can help your physician place a catheter on the right side of your body, the preferred location for a catheter since there are less complications.
The Surfacer System can help your physician place a catheter on the right side of your body, the preferred location for a catheter since there are less complications.- The Surfacer device works from the “inside-out” making it safer to pass through the venous obstruction and then enable a catheter to be inserted.
- A target is placed on the skin enabling your physician to see exactly where to point the device to exit the skin from the inside.
DEMONSTRATED RESULTS
- Results from 3 multicenter studies have shown the ability to achieve central venous access in over 95% of procedures where the Surfacer System is used.
- Total procedure time is typically less than 1 hour with the Surfacer System portion of the procedure usually taking less than 20 minutes.
- The Surfacer System has been used to place catheters in over 700 patients worldwide and has received authorization from the Food and Drug Administration (FDA) for use in the U.S.
- Medicare reimburses the procedure when performed in a hospital setting.
- To date there has been 13 papers published in medical journals reporting on the use of the Surfacer System.
POTENTIAL CLINICAL BENEFITS
 25% to 40% of patients with catheters develop central venous occlusions. The Surfacer System can help you to maintain catheter access to receive your therapy.
25% to 40% of patients with catheters develop central venous occlusions. The Surfacer System can help you to maintain catheter access to receive your therapy.- Use of the Surfacer System to obtain central venous access can help alleviate swelling of the arm and neck caused by venous occlusions.
- Avoiding placement of catheters on the left side by using the Surfacer System can improve the ability to create an arteriovenous (AV) fistula in your right arm, reducing the need for long-term use of a catheter and catheter-associated complications.
- The Surfacer procedure can be repeated again and again if your catheter fails or gets infected and you need to have it replaced.
ARE THERE RISKS ASSOCIATED WITH THE PROCEDURE?
- There are potential complications associated with Surfacer System procedure.
- These risks are similar to those associated with other procedures used to obtain central venous access.
- While rare, possible risks include pain, infection, bleeding, tissue or allergic reaction, pneumothrorax, pulmonary embolism, vessel vasospasm, vessel perforation, dissection, or aneurysm, embolization or thrombosis, arrhythmias, stroke, transient ischemic attack or nerve injury, development of arteriovenous fistula, or death.
- Your doctor will discuss these risks with you prior to the procedure.
For more information about managing thoracic central venous occlusions or the Surfacer® System, contact your healthcare professional or send us a message using our contact form.

